Abbott/Thoratec Corp recall HeartMate II and HeartMate 2 Left Ventricular Assist System (LVAS) due to long-term buildup causing obstruction.
See FDA Recall dated 04/15/2024


LVAD is a mechanical pump that helps the heart’s left ventricle circulate blood throughout the body. This device is used primarily by patient who suffer from severe heart failure and need additional support since their heart is not strong enough to pump blood on it’s own. These devices are used by patients waiting for a heart transplant, patients who are not eligible for a heart transplant, and, in some cases they can be used temporarily to support heart function while the heart/body records from an injury or surgery.
This recall is a Class 1 Recall, which is considered the most severe type of FDA Recall, having a potential for serious injury or death
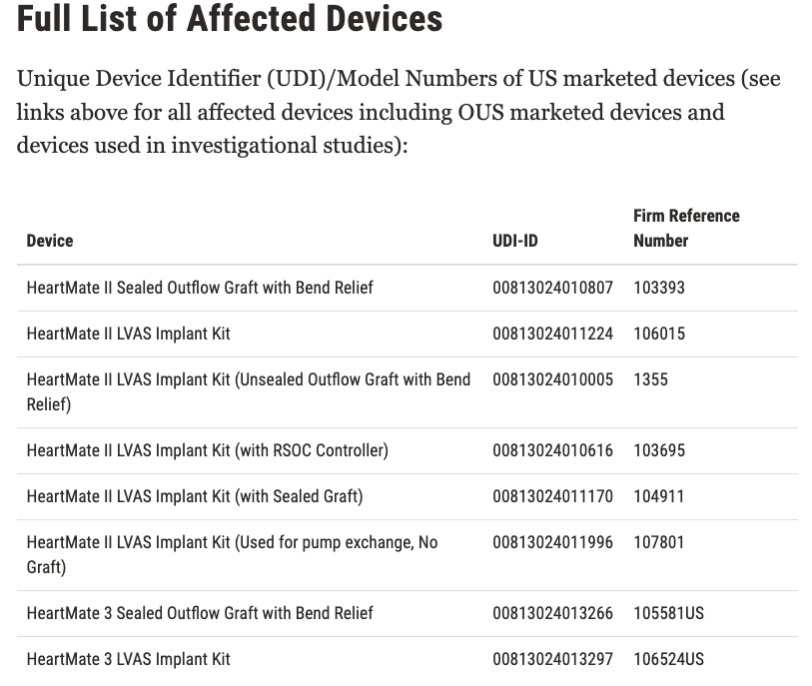
The affected devices were distributed from April 21 2008, and there have been around 13,883 devices recalled in the US. Here is the list of all affected devices:
Both of these devices are used to support patients with advanced heart failure. Demographic includes:
- Elderly Population: The risk of heart failure increases with age, ranging from 65 years and older)
- Men: While men are usually at a higher risk of suffering from heart failure at an earlier age, women in older age groups are also affected.
- Race & Ethnicity: Heart Failure has a higher prevalence among African Americans, Hispanics, and Latinos.
Risk Factors for Heart Failure include
- Hypertension: High blood pressure is a significant risk factor for heart failure and is more prevalent in certain populations, such as African Americans.
- Coronary Artery Disease: A history of heart attacks or coronary artery disease is a common precursor to heart failure.
- Diabetes: Patients with diabetes are at increased risk of developing heart failure.
- Obesity: Overweight and obese individuals are at higher risk of heart failure due to the increased strain on the heart.
Comorbidities
- Chronic Kidney Disease: Patients with kidney disease often have concurrent heart failure.
- Chronic Obstructive Pulmonary Disease (COPD): Respiratory conditions can exacerbate heart failure symptoms.
Lifestyle Factors
- Smoking: Smoking is a major risk factor for the development of heart failure.
- Sedentary Lifestyle: Lack of physical activity contributes to the risk of heart failure.
Geographic Variations
- Urban vs. Rural: There may be variations in heart failure prevalence and outcomes between urban and rural areas due to differences in access to healthcare and lifestyle factors.
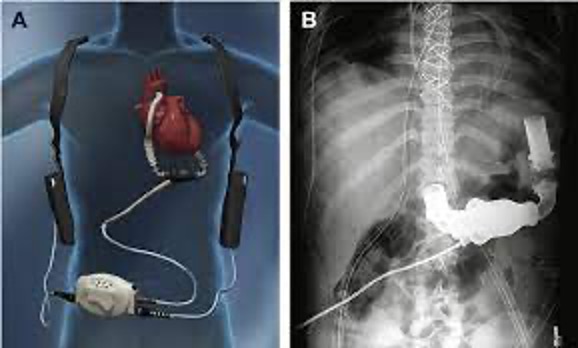
Both of these devices are used the help the heart pump blood when it’s not able to do it on it’s own. They can also be used for a short term, or a longer term for patients that are on the waiting list for a heart transplant. There are times where these devices can be used AFTER a heart transplant to help the heart and body to recover.
These devices are surgically implanted inside the chest, one end is attached to the left ventricle, which is the main pumping chamber of the heart, while the other is connected to the aorta, which is the main artery that carries blood from the heart to the rest of the body. When the heart is too weak to pump blood effectively, the pump takes over and draws blood from the left ventricle and pushes it into the aorta, ensuring that the blood circulates throughout the body. The device is powered by an external battery, which the patient must wear with them at all times.
The recall is due to Extrinsic Outflow Graft Obstruction (EOGO), which is a blockage of the outflow graft of the device. This outflow graft is the rube that carries blood from the LVAD to the aorta. This obstruction can happen when something (biological material) causes a blockage, which hinders the flow of blood from the LVAD to the aorta. According to the FDA, the “accumulation of biological material typically occurs over two years or more”
EOGO can lead to several complications due to the impaired blood flow, some of the complications include:
- Reduced Cardiac Output: blockages can reduce the amount of blood flow pumped by the device, which leads to inadequate circulation and reduced oxygen to the body’s tissues and organs.
- Heart Failure Symptoms: including fatigue, shortness of breath, swelling in the legs and abdomen (edema).
- Organ Disfunction: inadequate blood flow can cause dysfunction to vital organs, such as the kidneys, liver and brain; which can lead to acute kidney injury, liver congestion and cognitive issues
- Hemodynamic Instability: increased risk of arrhythmias and other cardiovascular problems
- Increased Risk of Blood Clots: irregular blood flow around the obstruction can promote the formation of blood clots, which increases the risk of stroke and other thromboembolitic events
- Pump Thrombosis: obstruction can also cause the blood to fester inside the LVAD, which increased the risk of blood clots forming inside the pump, which can lead to pump malfunction.
- Increased Risk of Infections: Any of the above that requires additional medical or surgical interventions increase the risk of infection.
There have been 273 reported injuries and 14 reports of death associated with EOGO and these devices
The Thoratec Corporation sent out a letter on 02/19/2024 to all affected customers asking them to “pay attention to low flow alarms, as this is the first symptom of significant outflow obstruction.”
According to an article posted by CBS on 04/16/2024: “The recall comes years after surgeons say they first noticed problems with the HeartMate II and HeartMate 3, manufactured by Thoratec Corp., a subsidiary of Abbott Laboratories.”
Sources:
- National Library of Medicine Article “Advanced Heart Failure Epidemiology and Outcomes: A Population-Based Study”
- JACC Journal: “Advanced Heart Failure Epidemiology and Outcomes: A Population-Based Study”
- National Library of Medicine Article “Who has Advanced Heart Failure? Definition and Epidemiology”
- JAMA Network Article “Demographic and Regional Trends of Heart Failure-Related Mortality in Young Adults in the US, 1999-2019”
- JACC Journals “Race and Ethnicity in Heart Failure: JACC Focus Seminar”
- American Heart Association “Risks for Heart Failure”